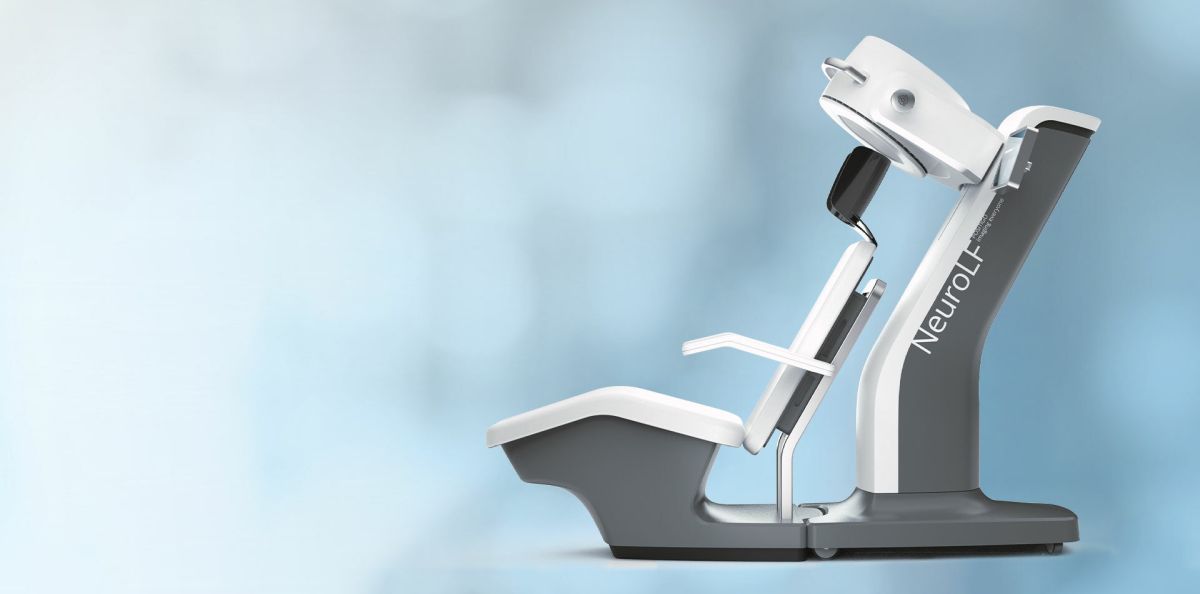
Positrigo prepares for the US launch of its dedicated brain PET system NeuroLF by incorporating its US subsidiary. The US is the most important market for Positrigo due to the availability of disease-modifying therapies for Alzheimer’s patients and the reimbursement of brain PET scans. The company expects regulatory approval in the first half of 2024.
Positrigo, a Swiss based company developing nuclear medical imaging devices to advance functional brain imaging incorporated its wholly owned US subsidiary. The US market experiences great momentum for brain Positron Emission Tomography (PET) due to the availability of disease-modifying therapies for Alzheimer’s disease (AD) and the recent announcement by the U.S. Centers for Medicare & Medicaid Services (CMS) to reimburse non-invasive imaging tests called amyloid PET.
Improved Amyloid PET reimbursement in the US
The U.S. CMS announced on October 13, 2023 that it has lifted its coverage limit of one beta-amyloid PET scan per lifetime for patients with AD. This decision will significantly increase accessibility to brain PET scans and comes in the wake of recent U.S. Food and Drug Administration (FDA) approvals of the first drugs to treat AD – monoclonal antibodies that target brain amyloid plaque deposits. Medicare coverage decisions will now be made by local Medicare Administrative Contractors (MACs).
Ron Lissak has been appointed as Executive Vice President and General Manager of Positrigo Inc. He is a medical imaging veteran and brings a wealth of experience in the nuclear medicine arena and an ever-growing network to Positrigo.
Regulatory approval in 2024
Positrigo is expecting regulatory approval in Europe and the US in the first half of 2024. Meanwhile several letters of intent have been signed by clinics which want to make sure to be among the first centers to receive the NeuroLF system.
Satisfying preliminary data
In June the company reported preliminary performance data of its dedicated brain PET which are well received by clinicians. Despite the minimalistic design and an attractive price-value offering, performance data indicate non-inferiority of Positrigo’s NeuroLF compared to conventional, room filling and more expensive PET/CT devices.
The tests have been performed at the University of Leipzig Medical Center University of Leipzig Medical Center Department of Nuclear Medicine in Germany. Prof. Dr. med. Sabri and Prof. Dr. med. Barthel at the Department of Nuclear Medicine in Leipzig were satisfied with the preliminary image quality data and spatial resolution of NeuroLF: “The first data show that NeuroLF produces similar images compared to a conventional digital PET/CT and is not inferior in comparison to such more expensive devices.” This conclusion has been supported by other experts in the field who had a chance to preview first image quality data of NeuroLF.
(Press release / SK)























































Please login or sign up to comment.
Commenting guidelines