Mimix Biotherapeutics has closed a multimillion Swiss franc investment round by onboarding strategic MedTech industry-savvy investors from Asia and Europe. The capital will enable the clinical translation of FastSkin treatment, a novel treatment method for chronic and acute wound management, and entry into the US market in H2 2024.
Since 2019, mimiX has been leveraging Sound Induced Morphogenesis (SIM), a proprietary technology that uses sound in conjunction with cells, stem cells, spheroids, organoids and bioactives, to create tissue-relevant architectures (bio-patterns). SIM replicates nature’s design strategy by controlling shape and function, the two fundamentals in developmental biology.
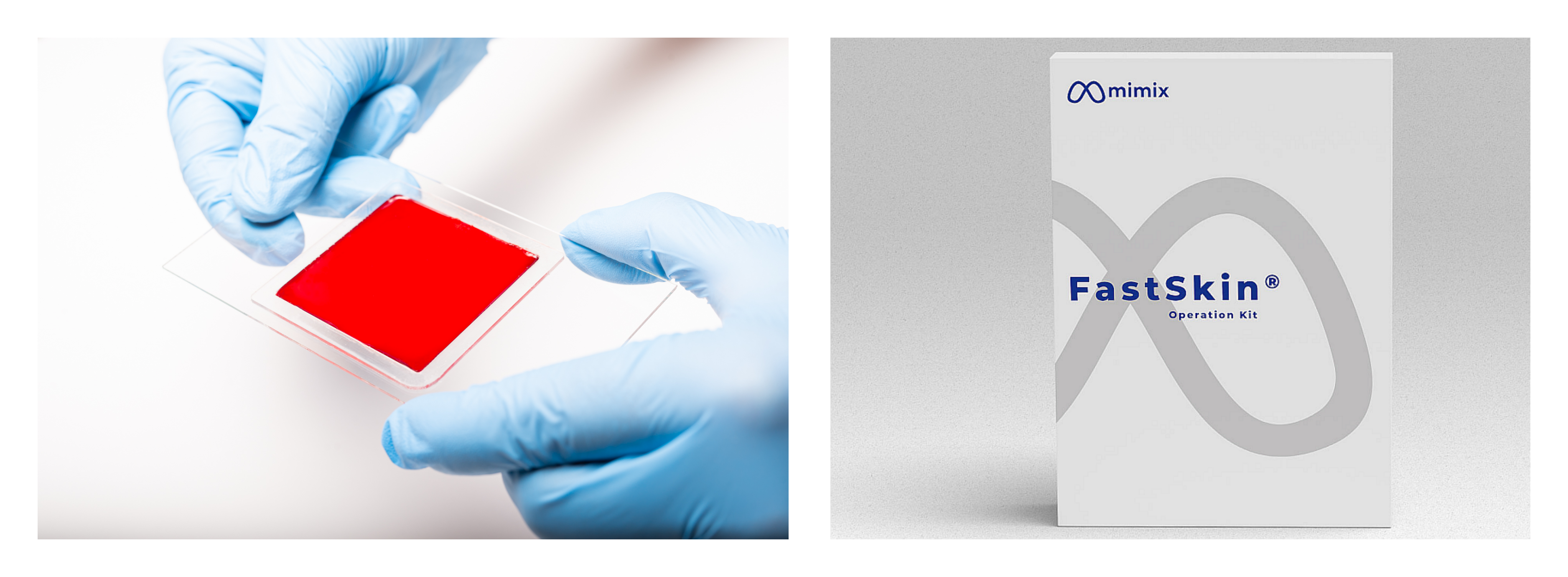
MimiX is focusing its energies on clinical translation with its first product, FastSkin, an advanced dermal substitute designed to treat acute and chronic wounds using the cutting-edge bio-fabrication technique of SIM. FastSkin has the potential to significantly improve patient outcomes and reduce healthcare costs.
The completion of the funding marks a significant milestone for mimiX, as it moves closer to achieving its mission of improving chronic wound management. Prominent industrial investors participated in mimiX’s financing round – these include Heraeus Group, an existing investor, and new investor Asia Jetway Pte, a Singapore-based holding producing and commercializing healthcare solutions in South East Asia through its affiliate companies LandMover, Shangai Keyman, Lader, Healthy Medical, and Jiangsu Lekaibio. Strategic investors joined the round to support mimiX in addressing a highly unmet need.
"We are thrilled to have the support of Heraeus Group and Asia Jetway, as we work to bring our innovative wound treatment to market," said Marc Thurner, CEO of MimiX. "Their expertise and resources will be invaluable to accelerate go-to-market and internationalization plans.”
The investment will accelerate the development and commercialization of FastSkin®, including the market entry into the US market in the second half of 2024. Headquartered in the Swiss Innovation Park in Biel/Bienne, Mimix is currently seeking US FDA approval.
(Press release/RAN)























































Please login or sign up to comment.
Commenting guidelines